About Small G: A Summer Idyll By Patricia Highsmith, About Small G
Search databaseBooksAll DatabasesAssemblyBiocollectionsBioProjectBioSampleBioSystemsBooksClinVarConserved DomainsdbGaPdbVarGeneGenomeGEO DataSetsGEO ProfilesGTRHomoloGeneIdentical Protein GroupsMedGenMeSHtravelhome.vn Web Sitetravelhome.vn CatalogNucleotideOMIMPMCPopSetProteinProtein ClustersProtein Family ModelsPubChem BioAssayPubChem CompoundPubChem SubstancePubMedSNPSRAStructureTaxonomyToolKitToolKitAllToolKitBookgh
travelhome.vn Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Đang xem: Small g: a summer idyll
Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999.
By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.

Correspondence to Eric J. Nestler, Division of Molecular Psychiatry, Departments of Psychiatry and Neurobiology, Yale University School of Medicine and Connecticut Mental Health Center, 34 Park Street, New Haven, Connecticut 06508.
In addition to the heterotrimeric G proteins, other forms of G proteins play important roles in cell function. These proteins belong to a large superfamily often referred to as “small G proteins” based on their low Mr (20,000 to 35,000) <24,25>. The small G proteins, like the heterotrimeric G proteins, bind guanine nucleotides, possess intrinsic GTPase activity and cycle through GDP- and GTP-bound forms (as shown in Fig. 20-1). One unifying feature of the various classes of G protein is that the binding of GTP versus GDP dramatically alters the affinity of the protein for some target molecule, apparently by inducing a large conformational change. Small G proteins appear to function as molecular switches that control several cellular processes. Examples of small G proteins and their possible functional roles are given in Table 20-2.
Table 20-2
Examples of Small G Proteins.
The best characterized small G protein is the Ras family, a series of related proteins of ~21 kDa
Ras proteins were identified originally as the oncogene products of Harvey and Kirsten rat sarcoma viruses. Subsequently, normal cellular homologues, also known as proto-oncogenes, of viral Ras were identified. Mammalian Ras proteins are encoded by three homologous genes: the photo-oncogene for Harvey Ras virus (H-ras), the proto-oncogene for Kirsten Ras virus (K-ras) and neural Ras (N-ras), although all three forms of Ras are found in diverse mammalian tissues, including brain. In addition, all three forms of Ras are membrane-associated proteins <24,25>.
The activity of Ras is highly regulated by a variety of associated proteins, as shown in Figure 20-3 <24–26>. Guanine nucleotide exchange factors (GEFs) stimulate the release of GDP from inactive Ras, which facilitates the binding of GTP. Thus, GEFs increase the activity of Ras. Multiple forms of GEFs have been identified, some specific for Ras and others for different small G proteins. In contrast, GAPs bind to Ras and activate its intrinsic GTPase activity, thereby reducing the functional activity of Ras. There also may be GTPase-inhibitory proteins (GIPs) that bind Ras and inhibit this GTPase activity, although these remain poorly described.
Xem thêm: Bánh Mì Pate For Banh Mi – Pate For Authentic Vietnamese Banh Mi
The analogy between Ras and its related proteins, on the one hand, and heterotrimeric G proteins and their related proteins, on the other, is striking (Fig. 20-3). G protein-coupled receptors essentially function as GEFs for the heterotrimeric G proteins, whereas RGS proteins and βγ subunits function as GTPase-activating and -inhibitory proteins. One major difference between the systems is that the intrinsic GTPase activity of Ras is far lower than that of heterotrimeric G protein α subunits. As a result, GAPs exert a much more profound effect on the functioning of Ras, essentially turning it on and off.
Upon binding GTP, there is a major conformational change in Ras, which is thought to be responsible for its functional activation. Ras has long been known to be a major control point for cell growth. Some of the cellular targets through which Ras produces its myriad effects have been identified. Numerous types of cell signal, including many and perhaps most growth factors, converge on Ras to regulate MAP-kinase pathways and, thereby, produce their diverse effects on cell function (see Chaps. 10 and 24) <24,25>. Briefly, it appears that activation of growth factor receptors results in the activation of a GEF, termed Sos, which in turn activates Ras. Activated Ras then binds to the N-terminal domain of a protein kinase called Raf, the first protein kinase in the MAP-kinase pathway. Ras appears to activate Raf via an indirect mechanism. Ras, which is membrane-bound, draws Raf to the plasma membrane, where it is activated through other means which are not yet completely understood. Anchoring of Ras in the plasma membrane may be mediated by isoprenylation, another point of analogy between Ras and heterotrimeric G proteins (Fig. 20-2). Ras exerts physiological effects via regulation of signaling pathways in addition to the MAP-kinase cascade. Although the mechanisms involved are not as well characterized, Ras has been shown to bind and perhaps regulate phosphatidylinositol-3-kinase, another protein involved in mediating the actions of growth factors, as well as certain isoforms of protein kinase C (see Chap. 21).
Although the major mechanism governing Ras activation is through growth factors, as outlined above, Ras function can be modulated by heterotrimeric G protein and second-messenger pathways <24,25>. The ability of free βγ subunits, particularly those released from Gi, to activate Ras was mentioned earlier. In addition, the cAMP cascade inhibits Ras activity in several systems, although it is unknown whether this occurs via direct phosphorylation by cAMP-dependent protein kinase of Ras or a closely associated protein. The “cross-talk” among these various intracellular cascades emphasizes that the multitude of intracellular messengers do not operate independently of one another but rather are highly integrated to coordinate the response of a cell to a myriad of extracellular signals.
Rab is a family of small G proteins involved in membrane vesicle trafficking
Mammalian tissues contain around 30 forms of Rab, which specifically associate with the various types of membrane vesicles and organelles that exist in cells <27,28>. Rab proteins, named originally as ras-related proteins in brain, are isoprenylated, which directs their association with membranes, as seen with isoprenylation of Ras and G protein γ subunits. However, unlike these other G proteins, the GTP- and GDP-bound forms of Rab appear to regulate association with the membrane compartment.
Xem thêm: Hệ Thống Buffet 99K Thoại Ngọc Hầu Cn5, Buffet Khói 222 Thoại Ngọc Hầu Cn5
Subtypes of Rab, particularly Rab3, have been implicated in the regulation of exocytosis and neurotransmitter release at nerve terminals <27–28a>. One possible scheme by which this occurs is shown in Figure 20-4. In its GTP-bound form, Rab is associated with synaptic vesicles, where it interacts with other membrane proteins to create a complex unfavorable for vesicle docking and perhaps fusion. Upon depolarization of the nerve terminal, a Rab GAP is activated, which results in dissociation of the GDP form of Rab from the vesicle membrane. This enables the synaptic vesicle to proceed with docking and fusion. The mechanism by which depolarization leads to activation of a Rab GAP and subsequently to release of Rab from the vesicle remains unknown, but Ca2+ is believed to be involved. To complicate matters further, several other proteins are involved in this Rab-synaptic vesicle cycle, including a Rab GDP dissociation inhibitor, which regulates the ability of Rab to bind to synaptic vesicles, and rabphilin, one of several synaptic vesicle proteins which can bind Rab (see Chap. 9).
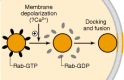
Figure 20-4
Schematic illustration of the proposed role of Rab in neurotransmitter release. Under basal conditions, GTP is bound to Rab3, which allows Rab3 to associate with other proteins on synaptic vesicles. This creates conditions unfavorable for the docking (more…)





Bình luận